Bacteriophages are modern antimicrobials containing a complex of natural antibacterial agents that have the ability to affect only harmful bacteria.
Manufactured by MicroGen
How does bacteriophages work?
All creatures living on earth have microscopic parasites-viruses. There are viruses and bacteria. The cycle of reproduction of bacterial viruses inevitably ends with the death of the microbe. To emphasize this feature, one of the discoverers of this effect, Felix D'erel, came up with a special name - "bacteriophages", in Greek - "bacteria eaters".
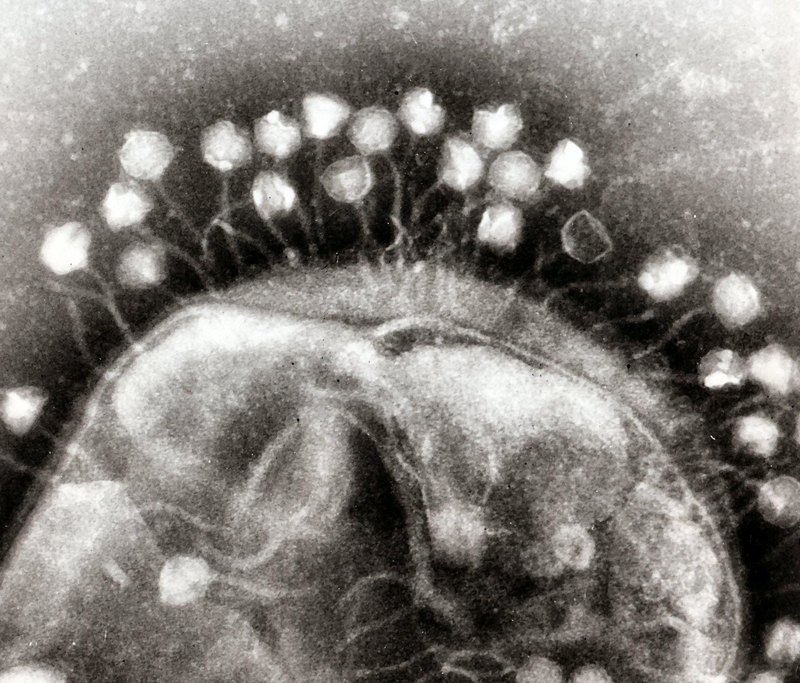
A photo taken with an electron microscope shows the process of fixing bacteriophages (coliphages T1) on the surface of the bacterium E. coli.
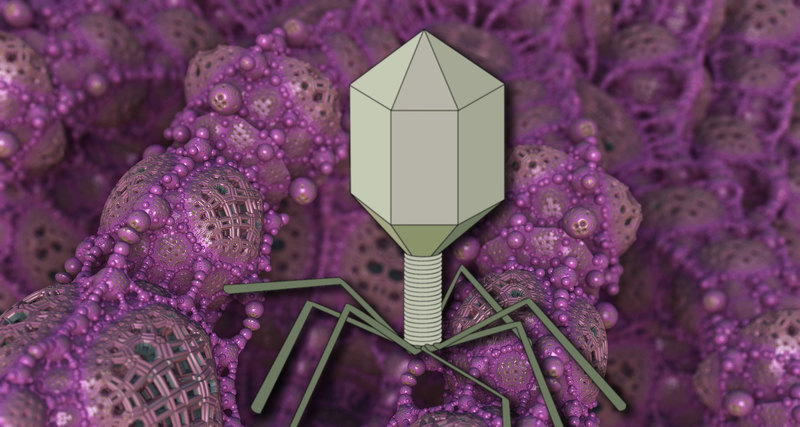
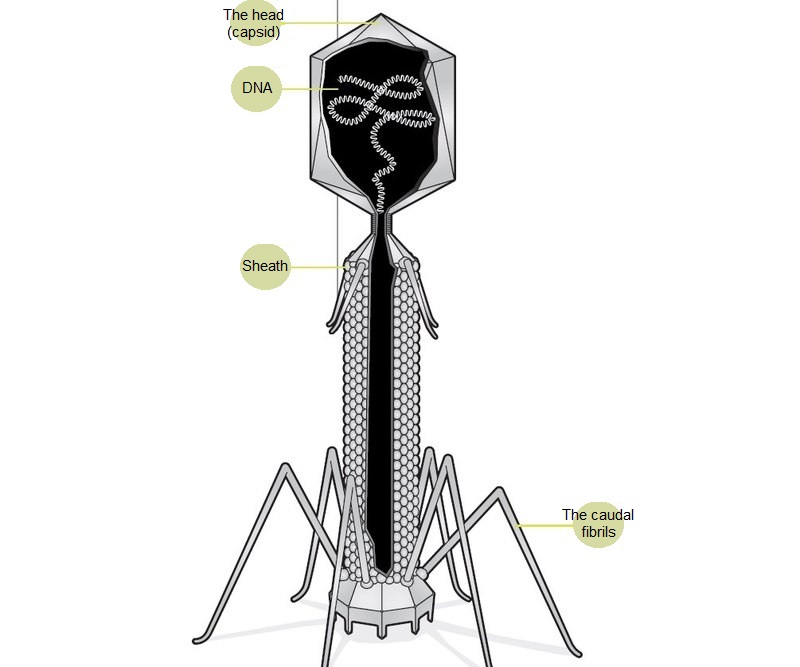
At the end of the twentieth century, it became clear that bacteria certainly dominate The earth's biosphere, accounting for more than 90% of its biomass. Each species has many specialized types of viruses. According to preliminary estimates, the number of bacteriophage species is about 1015. To understand the scale of this figure, we can say that if every person on Earth will discover one new bacteriophage every day, then it will take 30 years to describe all of them. Thus, bacteriophages are the most poorly studied creatures in our biosphere. Most of the bacteriophages known today belong to the order Caudovirales — tailed viruses. Their particles range in size from 50 to 200 nm. The tail of different length and shape ensures the attachment of the virus to the surface of the host bacterium, the head (capsid) serves as a repository for the genome. Genomic DNA encodes the structural proteins that form the" body " of the bacteriophage and the proteins that ensure the reproduction of the phage within the cell during infection. We can say that bacteriophage is a natural high-tech nanoobject. For example, phage tails are a "molecular syringe" that pierces the wall of a bacterium and, shrinking, injects its DNA inside the cell.
Bacteriophages for reproduction use the device of a bacterial cell, "reprogramming" it for production of new copies of viruses. The last stage of this process is lysis, the destruction of bacteria and the release of new bacteriophages.
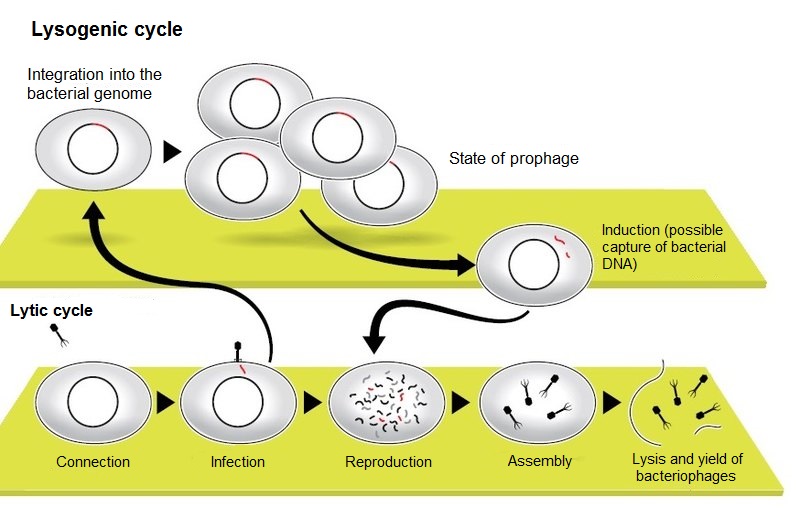
At this point, the infectious cycle begins. Its further stages consist of switching the mechanisms of life of the bacterium to the service of the bacteriophage, reproduction of its genome, the construction of multiple copies of viral membranes, packaging them in the DNA of the virus and, finally, the destruction (lysis) of the host cell. Each stage has many nuances that have a deep evolutionary and ecological meaning. After all, bacteria and their viral parasites coexist for hundreds of millions, if not billions of years. And this struggle for survival did not end with the total destruction of unicellular, nor the acquisition of total resistance to phages and uncontrolled reproduction of bacteria. In addition to the constant evolutionary competition of defense mechanisms in bacteria and attack in viruses, the reason for the balance can be considered and the fact that bacteriophages specialized in their infectious action. If there is a large colony of bacteria, where their victims will find the next generation of phages, the destruction of bacteria lytic (killing, literally — dissolving) phages is fast and continuous. If there are not enough potential victims or external conditions are not too suitable for effective reproduction of phages, then phages with a lysogenic development cycle gain an advantage. In this case, after the introduction of DNA into the bacterium, the phage does not immediately start the mechanism of infection, but for the time being exists inside the cell in a passive state, often penetrating into the bacterial genome. In this state the prophage virus can exist for a long time, passing together with the chromosome of the bacterium cycles of cell division. It is only when the bacterium enters a breeding environment that the lytic cycle of infection is activated. At the same time, when the DNA of the phage is released from the bacterial chromosome, adjacent portions of the bacterial genome are often captured, and their contents can then be transferred to the next bacterium that the bacteriophage infects. This process (gene transduction) is considered to be the most important means of transferring information between prokaryotes — organisms without cell nuclei.
A photo taken with an electron microscope shows the process of fixing bacteriophages (coliphages T1) on the surface of the bacterium E. coli.
All these molecular subtleties were not known in the second decade of the twentieth century, when "invisible infectious agents that destroy bacteria"were discovered. But even without the electron microscope, with which in the late 1940s for the first time it was possible to obtain images of bacteriophages, it was clear that they are able to destroy bacteria, including pathogens. This property was immediately demanded by medicine. The first attempts to treat phages of dysentery, wound infections, cholera, typhus, and even plague were carried out carefully enough, and the success looked quite convincing. But after the start of mass production and use of phage drugs euphoria was replaced by disappointment. Very little was known about bacteriophages, how to produce, purify and apply their dosage forms. Suffice it to say that according to the results undertaken in the United States at the end of the 1920-ies of verification in many industrial phage preparations actually bacteriophages did not appear at all.
Virus attack
The problem with antibiotics
The second half of the twentieth century in medicine can be called the " era of antibiotics." However, even the discoverer of penicillin Alexander Fleming in his Nobel lecture warned that the resistance of microbes to penicillin occurs quite quickly. For the time being, antibiotic resistance was offset by the development of new types of antimicrobial drugs. But since the 1990s, it has become clear that humanity is losing the "arms race" against microbes. First of all, the uncontrolled use of antibiotics is to blame not only for therapeutic but also for preventive purposes, and not only in medicine, but also in agriculture, the food industry and everyday life. As a result, resistance to these drugs began to develop not only in pathogenic bacteria, but also in the most common microorganisms living in soil and water, making them "conditional pathogens". Such bacteria comfortably exist in medical institutions, inhabiting plumbing, furniture, medical equipment, sometimes even disinfectant solutions. In people with weakened immune systems, which in most hospitals, they cause severe complications.
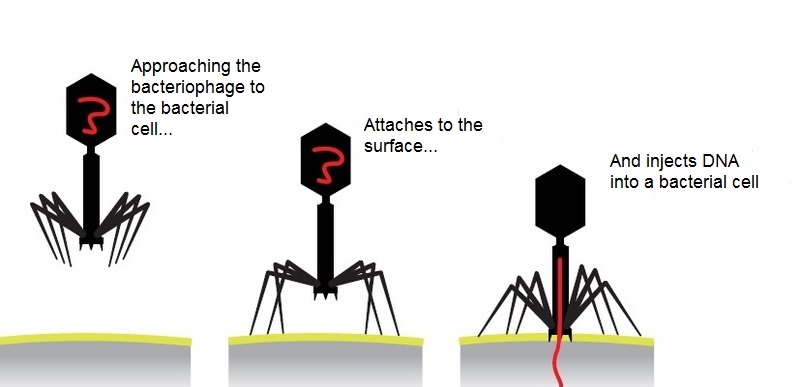
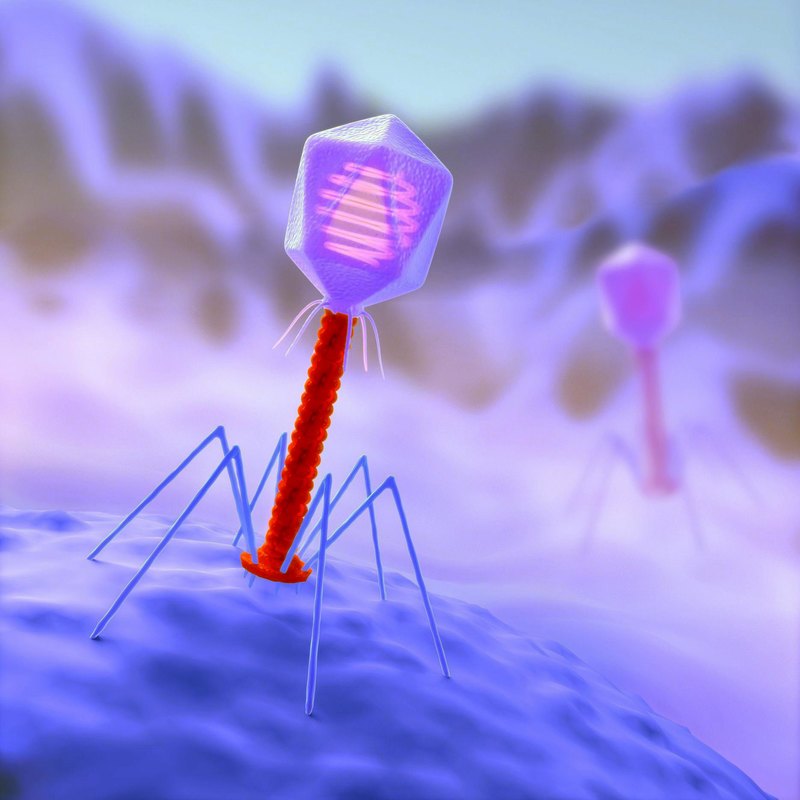
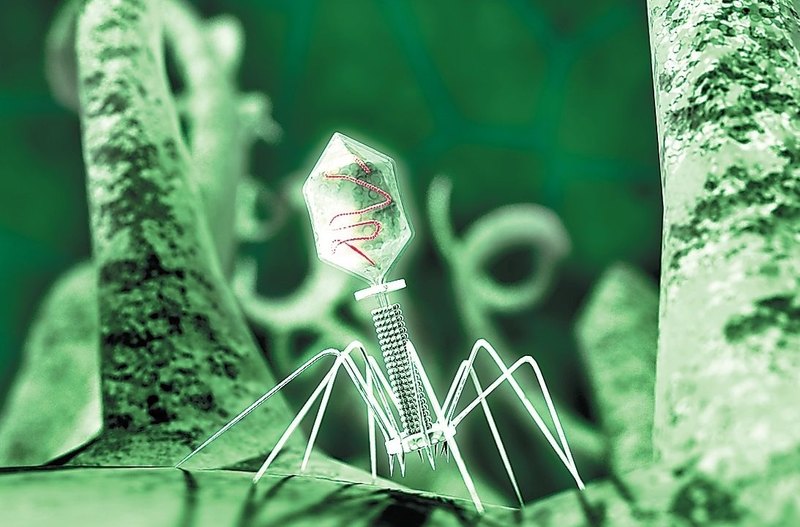
Bacteriophage is not a living creature, but a molecular nanomechanism created by nature. The tail of a bacteriophage is a syringe that pierces the wall of a bacterium and injects viral DNA that is stored in the head (capsid) inside the cell.
No wonder the medical community is sounding the alarm. In 2012, who Director General Margaret Chan issued a statement predicting the end of the era of antibiotics and the vulnerability of humanity to infectious diseases. However, the practical possibilities of combinatorial chemistry-the basis of pharmacological science-are far from exhausted. Another thing is that the development of antimicrobials is a very expensive process that does not bring such profits as many other drugs. So horror stories about "superbugs" - it is rather a warning, encouraging people to search for alternative solutions.
In the medical service
Advantages of bacteriophages as potential drugs are many. First of all-it is their myriad. Although it is also much easier to change the genetic apparatus of the bacteriophage than in bacteria, and even more so in higher organisms, this is not necessary. You can always find something suitable in nature. It is more about selection, consolidation of demanded properties and reproduction of the necessary bacteriophages. This can be compared with the breeding of breeds of dogs-sled, guard, hunting, hounds, fighting, decorative... They all remain dogs, but optimized for a certain type of action needed by man. Secondly, bacteriophages are strictly specific, that is, they destroy only a certain type of microbes, without inhibiting the normal human microflora. Third, when a bacteriophage finds a bacterium that it must destroy, it begins to multiply in the course of its life cycle. Thus, the question of dosage becomes not so acute. Fourth, bacteriophages do not cause side effects. All cases of allergic reactions when using therapeutic bacteriophages were caused either by impurities from which the drug was insufficiently purified, or by toxins released during the mass death of bacteria. The last phenomenon ,the "Herksheimer effect", is often observed in the application of antibiotics.
Two sides of the coin
Unfortunately, the shortcomings of medical bacteriophages are also many. The most important problem stems from the dignity-high specificity of phages. Each bacteriophage infects a strictly defined type of bacteria, not even a taxonomic species, but a number of narrower varieties, strains. Relatively speaking, as if the guard dog began to bark only at the two-meter-tall thug dressed in black raincoats, and did not react to the teenager in shorts climbing into the house in any way. Therefore, for current phage drugs, cases of ineffective use are frequent. A drug made against a certain set of strains and perfectly treating streptococcal angina in Smolensk may be powerless against all the signs of the same angina in Kemerovo. The disease is the same, caused by the same microbe, and the strains of Streptococcus in different regions are different.
Since bacteriophages in nature are innumerable and they constantly enter the human body with water, air, food, the immune system simply ignores them. Moreover, there is a hypothesis about the symbiosis of bacteriophages in the intestine, regulating the intestinal microflora. To achieve some kind of immune response is possible only with prolonged administration of large doses of phages into the body. But in this way you can achieve allergies to almost any substance. Finally, it is very important that bacteriophages are inexpensive. The development and production of a drug consisting of precisely selected bacteriophages with fully deciphered genomes, cultured according to modern biotechnological standards on certain strains of bacteria in chemically pure environments and highly purified, is much cheaper than for modern complex antibiotics. This allows for rapid adaptation of phagotherapeutic drugs to changing sets of pathogenic bacteria, as well as the use of bacteriophages in veterinary medicine, where expensive drugs are not economically justified.
For the most effective use of bacteriophage, an accurate diagnosis of the pathogenic microbe, up to the strain, is necessary. The most common method of diagnosis — culture seeding-takes a lot of time and does not give the required accuracy. Rapid methods-typing using polymerase chain reaction or mass spectrometry-are being implemented slowly due to the high cost of equipment and higher requirements for the qualification of laboratory technicians. Ideally, the selection of phage-components of the drug could be done against the infection of each individual patient, but it is expensive and in practice unacceptable.
Another important drawback of phages is their biological nature. In addition to the fact that bacteriophages require special storage and transportation conditions to maintain infectivity, this method of treatment opens up space for many speculations on the topic of "foreign DNA in humans". And although it is known that the bacteriophage in principle can not infect a human cell and introduce its DNA into it, it is not easy to change public opinion. From the biological nature and quite large, compared with low-molecular drugs (the same antibiotics), the size follows the third limitation-the problem of delivery of the bacteriophage to the body. If a microbial infection develops where the bacteriophage can be applied directly in the form of drops, spray or enema — on the skin, open wounds, burns, mucous membranes of the nasopharynx, ears, eyes, large intestine — then there are no problems.
But if the infection occurs in the internal organs, the situation is more complicated. Cases of successful treatment of kidney or spleen infections with conventional oral administration of bacteriophage are known. But the mechanism of penetration of relatively large (100 nm) phage particles from the stomach into the bloodstream and internal organs is poorly understood and varies greatly from patient to patient. Bacteriophages are also powerless against those microbes that develop inside cells, such as pathogens of tuberculosis and leprosy. Through the wall of the human cell bacteriophage can not get through.
It should be noted that the use of bacteriophages and antibiotics for medical purposes should not be contrasted. With their combined action, there is a mutual strengthening of the antibacterial effect. This allows, for example, to reduce antibiotic doses to values that do not cause pronounced side effects. Accordingly, the mechanism of development of resistance in bacteria to both components of the combined drug is almost impossible. Expanding the Arsenal of antimicrobial drugs gives more degrees of freedom in the choice of treatment methods. Thus, scientifically based development of the concept of bacteriophages in antimicrobial therapy is a promising direction. Bacteriophages are not so much an alternative as a Supplement and enhancement in the fight against infections.
Also please read:
Bacteriophage (From Wikipedia, the free encyclopedia)
Bacteriophages and Their Derivatives as Biotherapeutic Agents in Disease Prevention and Treatment
Bacteriophages - StatPearls - NCBI Bookshelf
Bacteriophages (article) | Viruses | Khan Academy
