Expiration date: 09/2026
The composition and form of issue:
Tablets set
Fluconazole, tablets. 1 tablet contains fluconazole 150 mg
other ingredients: microcrystalline cellulose, calcium phosphate dibasic (anhydrous) croscarmellose sodium magnesium stearate silica colloidal anhydrous, the dye "Ponceau 4R varnish"
Azithromycin, tablet, film-coated. 1 tablet contains azithromycin (as dihydrate) 1 g
excipients: sodium lauryl sulfate croscarmellose sodium povidone K30 magnesium stearate silica colloidal anhydrous (waterless)
shell: hypromellose diethyl magnesium silicate hydrate purified (talc) titanium dioxide macrogol 4000 dye "Ponceau 4R varnish"
The Secnidazole, tablets, film-coated. 1 tablet contains the Secnidazole 1 g
excipients: corn starch, microcrystalline silicon dioxide colloidal (anhydrous), sodium starch of glycolate povidone purified talc 30 magnesium stearate
shell: hydroxypropyl methylcellulose diethyl purified talc titanium dioxide macrogol 4000
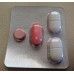
in the package contour 4 PCs (1 table. Fluconazole 1 tab. Azithromycin 2 table. Secnidazole) in the paper cartons 1 package.
Description pharmaceutical form:
1. Fluconazole. Round-valium pills pink color with a facet and valium. At the turn of pink color.
2. Azithromycin. Biconvex tablets capsuleline shape, covered with a shell pink color, with valium. On a break - white or almost white.
3. The Secnidazole. Capsuleline biconvex tablets, coated white or almost white, with valium. On a break — white or almost white.
Feature:
Fluconazole — antifungal agent ATC: J02AC01.
Azithromycin — an antibiotic of azalide ATC: J01FA10.
The Secnidazole — antimicrobial and Antiprotozoal medium ATX: P01AB07.
Pharmacokinetics:
1. Fluconazole. After oral fluconazole is well absorbed, its bioavailability is 90%. Cmax after ingestion on an empty stomach 150 mg is 90% of the content in the plasma when the/in the introduction in a dose of 2.5–3.5 mg/l. Simultaneous eating does not affect the absorption of drug taken orally. Concentration in plasma reaches a peak after 0.5–1.5 h after administration, the T1/2 of fluconazole is about 30 h. plasma Concentrations are directly proportional to the dose.
The apparent volume of distribution approaches total water content in the body. Tying with the plasma protein — 11-12%.
Fluconazole penetrates well into all body fluids organism. The drug concentration in saliva and sputum are similar to plasma levels.
In the stratum corneum, the epidermis, derma and sweat are reached high concentrations, that exceed the whey.
Fluconazole write mainly kidneys, about 80% of the administered dose appears kidneys in an unmodified form. Fluconazole clearance is proportional to creatinine clearance. Metabolites of fluconazole in peripheral blood was not detected.
2. Azithromycin. Rapidly absorbed from the gastrointestinal tract due to its stability in acidic medium and lipophilicity. Once inside the dose of 500 mg C max azithromycin in plasma achieved through 2.5–of 2.96 h and is 0.4 mg/l. Bioavailability is 37%.
Azithromycin well into the respiratory tract, organs and tissues of the urogenital tract (in particular, in the prostate gland), in skin and soft tissue. High concentration in tissues (10-50 times higher than in blood plasma) and long half-life due to low binding of azithromycin to plasma proteins of blood, as well as its ability to penetrate into eukaryotic cells and concentrate in an environment with low pH, environmental lysosomes. This in turn defines a large apparent volume of distribution (31.1 l/kg) and high plasma clearance. The ability of azithromycin to accumulate mainly in lysosomes is particularly important for elimination of intracellular pathogens. Proved that phagocytes deliver azithromycin to the places of infection, where it is released in the process of phagocytosis. The concentration of azithromycin in hotbeds of infection was significantly higher than in healthy tissues (on average 24-34%) and correlated with the degree of inflammatory edema. Despite the high concentration in phagocytes, azithromycin did not significantly impact on their function. Azithromycin remains in bactericidal concentrations in inflammation within 5-7 days after your last dose.
Demetiliruetsa in the liver, the resulting metabolites are not active.
Excretion of azithromycin from blood plasma is 2 phase: T1/2 is 14-20 hours in the range from 8 to 24 h after drug administration and 41 h — in the interval from 24 to 72 h.
3. The Secnidazole. Removals — high, bioavailability is 80%. Metabolized in the liver. Tmax after a single oral dose of 2 g — 4 hours Excreted by the kidneys — 72 h (16% of the dose). Secretiruetsa in breast milk, crosses the placental barrier.
Description pharmacological action:
1. Fluconazole. Fluconazole, representative of a class of triazole antifungal agents, is a powerful inhibitor selective synthesis sterols in the cell mushrooms.
The drug is effective in opportunistic mycoses, including caused by Candida spp. Cryptococcus neoformans, Microsporum spp., Trichophyton spp. Also shown is the activity of fluconazole on models of endemic mycoses, including infections caused by Blastomyces dermatitidis, Coccidioides immitis and Histoplasma capsulatum.
2. Azithromycin. The broad-spectrum antibiotic. Is a representative subgroup of macrolide antibiotics — azalidov. When you create in the inflammation high concentrations has a bactericidal effect.
To azithromycin sensitive gram-positive cocci: Streptococcus pneumoniae, St. pyogenes, St.agalactiae, streptococci groups C, F and G, Staphylococcus aureus, St. viridans gram-negative bacteria: Haemophilus influenzae, Moraxella catarrhalis, Bordetella pertussis, B. parapertussis, Legionella pneumophila, H. ducreyi, Campylobacter jejuni, Neisseria gonorrhoeae and Gardnerella vaginalis and some anaerobic microorganisms: Bacteroides bivius, Clostridium perfringens, Peptostreptococcus spp and Chlamydia trachomatis, Mycoplasma pneumoniae, Ureaplasma urealyticum, Treponema pallidum, Borrelia burgdorferi. Azithromycin is inactive against gram-positive bacteria resistant to erythromycin.
3.The Secnidazole. Antimicrobial bactericide - synthetic derivative of nitroimidazole. Active against obligate anaerobic bacteria (spore - and asporogenous), agents of some protozoal infections: Trichomonas spp., Giardia lamblia, Entamoeba histolytica. Inactive against aerobic bacteria. Increases the sensitivity of tumors to radiation exposure, causes sensitisation to alcohol (teturamopodobnaya action). Interacts with DNA and causes a disturbance of the spiral structure, the rupture of filaments, inhibition of the synthesis of nucleic acids and cell death.
Indications:
Co-infection of the urogenital tract, sexually transmitted diseases such as gonorrhea, trichomoniasis, chlamydiosis, bacterial vaginosis, fungal infections and accompanying specific and non-specific cystitis, urethritis, vulvovaginitis and cervicitis.
Contraindications:
- hypersensitivity to fluconazole (incl. other azole compounds), azithromycin (including to other macrolides), Secnidazole (including other nitroimidazoles)
- concomitant use of terfenadine, astemizole or other drugs that lengthen the QT interval
- organic diseases of the Central nervous system
- pregnancy
- lactation
- the children's age.
Caution: when administered simultaneously with cizapridom, rifabutin or other drugs metabolized by cytochrome system P450.
Side effects:
From the side of cardiovascular system: very rarely — violations heart rhythm.
From the gastrointestinal tract and liver: nausea, vomiting, diarrhea, dyspepsia, abdominal pain, flatulence, lack of appetite.
From the sensory organs: rare — violations of taste.
With the hematopoietic system: leukopenia.
Allergic reactions: skin rash, pruritus, anaphylactic reaction.
Other: rarely — headache, dizziness, stomatitis.
Drug interactions:
1. Fluconazole. In the application of fluconazole with warfarin increases PV (average 12%). In this regard, it is recommended to carefully monitor the performance of prothrombin time in patients receiving the drug in combination with coumarin anticoagulants.
Fluconazole increases the half-life from plasma oral gipoglikemicakih funds derived sulfonylureas (chlorpropamide, glibenclamide, glipizide, tolbutamide) in healthy people. The combined use of Fluconazole and oral hypoglycemic agents in diabetic patients is allowed, however, the physician should bear in mind the possibility of hypoglycemia.
Patients receiving high doses of theophylline or who have a likelihood of teofillinom of intoxication should be under observation for early detection of symptoms of overdose of theophylline, because of fluconazole leads to a decrease in the average speed of clearance of theophylline from plasma.
Contraindicated concomitant use of terfenadine, astemizole or other drugs that lengthen the QT interval.
Care must be taken when administered simultaneously with cizapridom, rifabutin or other drugs metabolized by cytochrome system P450.
2. Azithromycin. Antacids (aluminum and magnesium), ethanol and food slows down and reduces absorption.
The joint appointment of warfarin and azithromycin (in normal doses) changes in PV have not been identified, however, given that in the interaction of macrolides and warfarin may increase anticoagulant effect, patients requires careful monitoring of PV.
Digoxin — increase the concentration of digoxin.
Ergotamine/dihydroergotamine — increase the toxic effect (vasospasm, disesthesias).
Slows excretion and increases the plasma concentration and toxicity of cycloserine, indirect anticoagulants, methylprednisolone, felodipine, and drugs undergoing microsomal oxidation (carbamazepine, terfenadine, cyclosporine, gexobarbitala, ergot alkaloids, valproic acid, disopyramide, bromocriptine, phenytoin, oral gipoglikemicakie means, theophylline and xanthine derivatives etc.) is due to the inhibition of microsomal oxidation in hepatocytes by azithromycin.
Lincosamide weaken the effectiveness of tetracycline and chloramphenicol — strengthen.
3. The Secnidazole. Enhances hypoglycemic effect of insulin and oral antidiabetic drugs. Combined with alcohol can develop disulfiramopodobna reaction (abdominal cramps, nausea, vomiting, headache, rush of blood to the face).
Method of application and dose:
Inside, one. Take all 4 tables., included in the blister, taking into account the meal (because the absorption of azithromycin change at simultaneous food intake, it is better to take an hour before food or 2 hours after a meal).